Abstract

Conditioning protocols for patients undergoing allogeneic hematopoietic cell transplantation (allo-HCT) are being developed continuously to improve their anti-leukemic efficacy and reduce their toxicity, especially in older patients or those with comorbidities.
In the present study, we compared the conditioning protocol FluMel (fludarabine, median 150 mg/m2; melphalan, median 140mg/m2) with conditioning protocols based on FluMel with an additional alkylating agent i.e. either FBM (fludarabine, median 150mg/m2; carmustine 300-400mg/m2; and melphalan, median 110 mg/m2) or FTM (fludarabine, median 150mg/m2; thiotepa 5-10mg/kg; and melphalan, median 110 mg/m2). From the registry of the EBMT Acute Leukemia Working Party, we identified 1551 adult patients (1139 patients with FluMel and 412 patients with FBM/FTM) with acute myeloid leukemia (AML) with intermediate/poor cytogenetic risk in first complete remission (CR1), and transplanted with unmanipulated peripheral blood grafts from related or unrelated donors. Impacts of these two regimens on the outcomes were evaluated using Cox multivariable models.
Patients in the FBM/FTM group were older (62.4 years vs. 59.9 years, p<0.001), had a worse Karnofsky performance score (KPS<90, 31.2% vs. 22.7%, p<0.001) and received more often grafts from unrelated donors (77.9% vs. 66.6%, p<0.001), which were associated with worse overall survival (OS). Patients conditioned with FluMel more often received an in vivo T-cell depletion (TCD, 85 vs 90%, p<0.01) and this was based on alemtuzumab (21% vs. 69%) compared with patients conditioned with FBM/FTM, who received more frequently anti-thymocyte globuline (ATG, 78% vs. 7%).
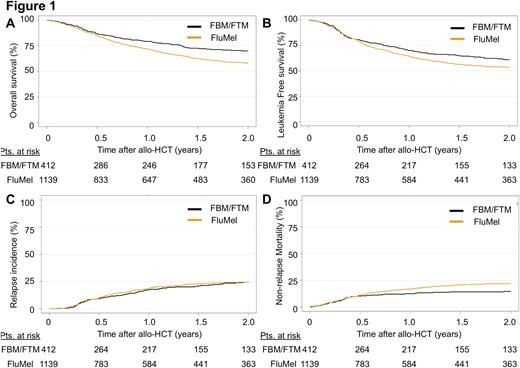
On univariate analysis, patients in the FBM/FTM group showed a better OS (2y OS: 69.9% vs. 57.9%, hazard ratio (HR) 0.62, p<0.001), leukemia-free survival (LFS) (2y LFS: 61% vs 53.5%, HR 0.74, p=0.004), and less non-relapse mortality (NRM) (2y NRM: 14.6% vs 22%, HR: 0.54, p<0.001) at 2 years compared to patients treated with FluMel. No significant differences were observed in relapse incidence (RI) (2y RI: 24.3% vs. 24.5%, HR: 0.95, p=0.73). Multivariate analysis confirmed improved outcomes (OS, LFS and NRM) in patients treated with FBM/FTM and no significant difference in RI. Differences in GvHD prophylaxis and in melphalan dose were observed in both cohorts, which might have influenced patient outcomes.
In conclusion, the addition of a second alkylating agent (BCNU/carmustine or thiotepa) to FluMel as FBM/FTM conditioning improves overall survival in AML patients with intermediate/poor risk cytogenetics in CR.
Disclosures
Duque Afonso:IPSEN: Honoraria; Riemser: Honoraria; AstraZeneca: Honoraria; Amgen: Honoraria; Roche: Consultancy, Honoraria; Lilly: Honoraria; SOBI: Honoraria. Finke:Riemser Pharma: Research Funding. Craddock:Novartis: Consultancy; Abbvie: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; JAZZ: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy. Bloor:Janssen: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau. Nicholson:Amgen: Other: Travel grant; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Meeting grant. Snowden:Medac: Honoraria; Novartis: Honoraria; Mallinckrodt: Honoraria; Gilead: Honoraria; Jazz: Honoraria; Janssen: Honoraria. Wagner:Amgen: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other: NA; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medac: Other: NA; Novartis: Membership on an entity's Board of Directors or advisory committees. Spyridonidis:Stemline/Menarini: Consultancy. Ciceri:Kite Pharma: Consultancy. Mohty:Jazz Pharmaceuticals: Honoraria, Research Funding; Celgene: Honoraria; Bristol Myers Squibb: Honoraria; Takeda: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Adaptive Biotechnologies: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal